Abstract
Introduction: Epstein Barr virus-positive (EBV+) diffuse large B- cell lymphoma (DLBCL), not otherwise specified (NOS) is a new entity recognized in the 4th edition of the 2016 classification of the WHO. Prevalence rates range from 5% in Western countries to 10-15% in Asia and South America. The IPI and Oyama score are prognostic factors for survival in this entity. The aim of this study is to evaluate the clinical characteristics and survival in EBV+ DLBCL, NOS.
Methods: The study was retrospective, reviewing clinical records of patients treated at Rebagliati Martins Hospital between years 2002 - 2017. Patients of both sexes greater than or equal to eighteen years of age with the diagnosis of EBV+DLBCL, NOS were included. IRB approval was obtained prior to research. Pathological samples were reviewed by hematopathologists at our institution to confirm the diagnosis. Pertinent clinicopathological data were collected through chart review and are presented using descriptive statistics. Overall survival (OS) was determinate according to the Kaplan -Meier method, the comparison of the survival curves were made with the log-rank test. Univariate and multivariate Cox proportional-hazard regression models were fitted to evaluate hazard ratios (HR) for OS.
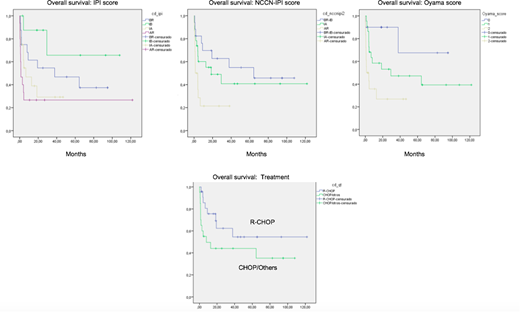
Results: A total of 60 patients with a diagnosis of EBV+DLBCL, NOS were included in this analysis. The median age at diagnosis was 70 years (range 25-95 years), 62 % of patients were older than 60 and there was a male predominance (65%). Activated B-cell phenotype (ABC) was present in 57% of patients. Clinically, 62% presented ECOG >1, 52% had elevated serum LDH, 57% had B symptoms, 67% had an extranodal disease and 55% had stage III/IV. IPI score distribution was low-risk in 28% of patients, low-intermediate in 16%, high-intermediate in 30% and high-risk in 26% of patients. The Oyama score distribution was low risk in 20 % of patients, intermediate risk 45 % and high risk in 35% of patients. NCCN-IPI score distribution was low-risk in 5% of patients, low-intermediate in 25%, high-intermediate in 42% and high-risk in 28% of patients. 51% of patients received R-CHOP, 29% received CHOP, and 20% received other regimens. The overall response rate was 56%; 44% had a complete response and 12% had a partial response. The 5-year OS rate was 38% with a median of 9 months. In the univariate analysis, ECOG 2-4 (p=0.001), stage III/IV (p=0.001), CHOP and others regimens (p=0.025) were associated with a poor prognosis. EBV+ DLBCL, NOS patients with low, low-intermediate, high-intermediate and high-risk IPI had 5-year OS rates of 47%, 66%, 29% and 27%, respectively (p=0.006). In the NCCN-IPI score, EBV+ DLBCL, NOS patients with low/ low-intermediate, high-intermediate and high-risk had 5-year OS rates of 55%, 41%, 21%, respectively (p=0.014). In the Oyama score, EBV+ DLBCL, NOS patients with low, intermediate and high-risk had 5-year OS rates of 68%, 47%, 27%, respectively (p=0.004) In the multivariate analysis, patients with stage III/IV had a worse outcome (HR 3.8, 95% 1.6-9.2; p=0.003). The IPI score (HR 2.6, 95%CI, 1.1-6.3), NCCN-IPI score (HR3.4, 95%CI, 1.3-8.6, and Oyama score (HR 6.8, 95%CI, 1.5-31.2) were significant in the high-risk group.
Conclusions: This is the largest Latin American cohort with EBV positive DLBCL, NOS reported so far. This entity is an aggressive tumor with a worse prognosis. IPI, NCCN-IPI and Oyama score are good prognostic index models for this disease. Rituximab appears to improve OS.
Castillo:Janssen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Millennium: Research Funding; Beigene: Consultancy, Research Funding; Genentech: Consultancy; Abbvie: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal